Biocompatibility evaluates how a medical device interacts with the body, including materials and any manufacturing or sterilization residues that could cause harmful biological responses during intended use.
A complete evaluation confirms that the finished device will not trigger irritation, sensitization or systemic effects. When this work is overlooked or incomplete, patients face preventable biological risks, and manufacturers face regulatory delays, test failures, redesigns and potential market withdrawal.
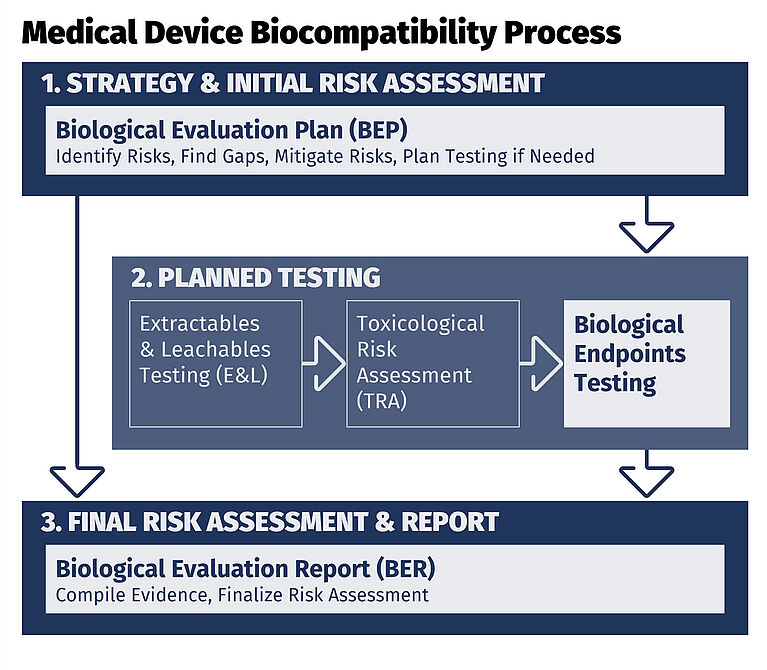
ISO 10993 requires a risk-based approach to determine what must be tested and what can be justified with existing data. A well-planned biocompatibility program helps build a defensible biological safety case for FDA, EU MDR and global submissions.